Free shipping on orders of $75+ for patients.
Doctors must be logged in to view professional pricing. $500+
You are viewing professional pricing.
Doctors must be logged in to view professional pricing. $500+
You are viewing professional pricing.




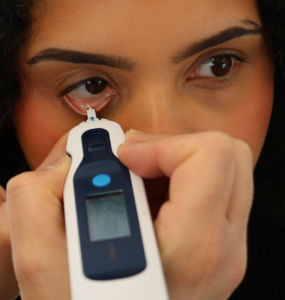


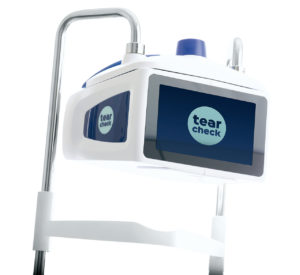
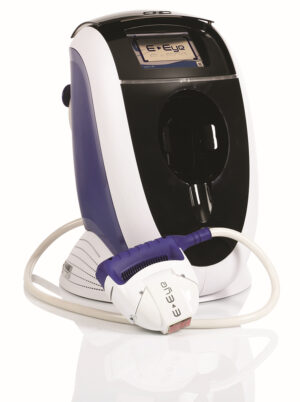
Reviews
There are no reviews yet.